This paper focused on DNA vaccinations and the history and prior research surrounding the topic. It presents two studies and the results from these previous studies. Taking into the account the history and study results, this paper urges for a continuation of research on DNA vaccinations.

Are DNA Vaccinations the Next Medical Breakthrough?
Jennifer Sanchez-Flores
English 21003
Writing for the Sciences
Professor Debra Williams
Abstract
DNA vaccinations are a relatively new topic with a need for more research. The research aims to further prove the efficiency of DNA vaccinations so it could be used to save many, of all ages, from viruses. Although it is more hypothetical at the moment, the highest goal is to design a DNA vaccination for cancer or HIV. Since it is still a relatively new topic, there is much to explore on this topic. The hypothesis that researchers hope will occur is that DNA vaccinations will prove to be superior to traditional vaccines. The two studies included in this paper have different subjects. The first one mentioned focused on children of different races and ethnicity randomly placed in groups. The second one mentioned focus on the same type of mice, also randomized. Both followed the same design. At least two groups were set up to compare and contrast the differences between the group receiving the DNA vaccination and the group who receives the regular form of treatment. They were both analyzed after the trial to test for immunity. The key data was different in both studies, however, both studies contained different subjects. While the study with children as subjects saw no significant difference, the study with mice did see a clear difference. Both were analyzed similarly, with T-tests, and concluded consistency in results. It was concluded that DNA vaccinations work, as they hypothesized, but at no significant difference. This is what most currently is concluded from these studies, but these studies are at its earliest studies. Future studies need to focus of the holes of the current research and change and manipulate certain variables so further research can show better results.
Introduction
Vaccinations are considered a breakthrough in medical history. Its introduction saved millions of lives from the smallpox virus due to Edward Jenner’s breakthrough of the vaccine in 1796, although, he wasn’t the first to introduce the idea or perform the procedure. Since this discovery, many vaccines were introduced and now are considered typical in all offices. Diseases once deadly and common are no longer a threat because of vaccines. Researchers have been looking for ways to improve traditional vaccines. As early as the 1950s, DNA vaccines have been constructed hypothetically and small experiments started occurring in the 1990s. Direct DNA injection allows for the production of an immune response because of the reproduction of the viral protein. More research should be conducted to determine whether it should be implemented into traditional settings. The construction of these DNA strands are now easier to develop into a vaccine since they have codon-optimized genes leading to antigenic protein production. (Lee 2018) Four DNA vaccines have currently been approved for veterinary use. They are the only approved DNA vaccines at the moment. More research can ensure DNA vaccinations come to traditional settings as well. Early studies proved its safety, but failed to show that it resulted in high immunity. This topic is still relatively new, but Whole vaccines rely on the host receiving the virus itself, DNA vaccines don’t. Extensive research needs to be conducted in order to determine whether the development and introduction of DNA vaccinations into standard usage by all primary care physicians should be carried out.
Methods
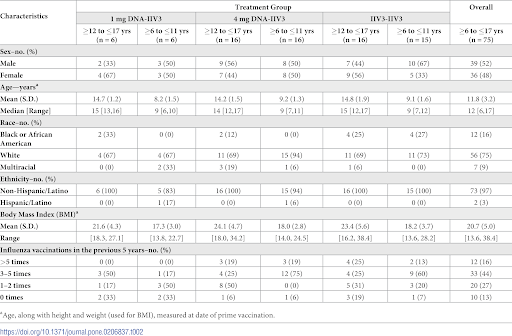
Houser, Yamshchikov, Bellamy et al., worked on a paper about a study focusing on the use of DNA vaccines on children. This study had children aged six to seventeen years old. Since this research was conducted on the influenza virus, its age choices were important since the flu vaccine is most prominently a main area of concern for parents. The study’s independent variables were valid as it included both types of treatment levels that are to be compared to test the effectiveness of DNA vaccinations and the same amount of children in each treatment level. The dependent variables are also valid as it tests the antibody response of the children in each treatment level group. Extraneous variables are present in the race and ethnicity. Although it was recorded, more thought should have been put into the importance of it. T-tests and non parametric tests were conducted after the results were obtained which found that results were consistent. Twelve children received the 1 mg DNA treatment, 32 children (which includes the twelve children who revived the 1 mg DNA treatment) received the 4 mg DNA treatment, and 31 children received the prime treatment. Furthermore, there isn’t an equal amount of representation from each race in each group. The treatment each child receives (DNA or traditional vaccines) was random so no bias was present. This study did include a large portion of subjects, however, considering the vast amount of children in the United States at risk for the influenza, the size should be bigger and include more black and Hispanic children since overall, the study includes 75% white children. White children live different lifestyles than black or Hispanic children and are genetically different. This study was similar to other studies involving the testing of DNA vaccinations. Each study attempts to discover a breakthrough, however, this study failed to further prove the efficiency of DNA vaccines.
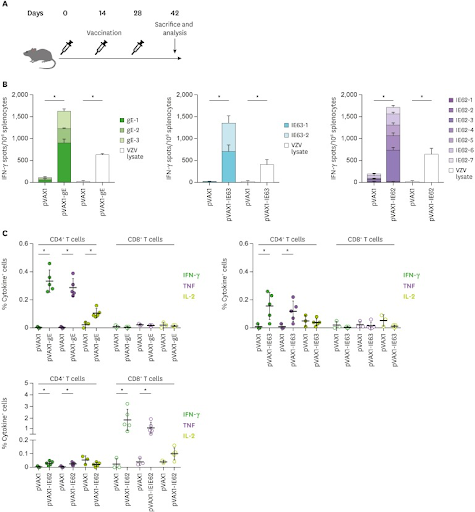
Most studies already completed on DNA vaccinations have been completed on animals. The only current approved DNA vaccinations are for animal use and its a relatively new study so its common for these studies to be completed on animals. Kim, Park, et al., focused on a study looking at the DNA vaccination for Herpes Zoster (HZ), or shingles, on female mice. The motivation behind this study was the effectiveness of the current HZ vaccine available. The vaccines efficiency decreases over time with age. Five to six-week-old female mice were obtained from Korea. Although this vaccination targets human, mice are tested on first, like most products or medicine targeted for humans. There were three treatment levels, along with a control group that, that these mice were randomly placed in, and all mice were the same kind of mice, so no bias was present in the completion of trial. There were five mice in each treatment level, except for the control group were there were only three mice present. More mice should have been studied on. A smaller number of subjects could result in inaccurate results. However, an increased amount of subjects leads to smaller chance of error because you are able to obtain much more information and results. The study concluded that all mice who received the DNA vaccination treatment had T-cell responses concluding the vaccinations safety and efficiency. For this study, the independent variables were the DNA vaccination. This study designed three different HZ DNA plasmids and 30 µg of each DNA plasma (pVAX1-gE, pVAX1-IE63, or pVAX1-IE62) was injected into each mouse. The dependent variables tested the immunity the mice had obtained from the virus. In the figure below, section B shows the development of immunity gained by these mice. Section C displays what specific type of response was given from the DNA vaccinations. For example, pVAX1-IE63 induced a CD4+ T-cell response, but not CD8+ T-cell response. No extraneous variables were shown when all mice were held in pathogen free environments prior to the study. Finally, on the figure below, standard deviation bars are present to show the consistency in results and t-tests were conducted on the data and they concluded that the differences between the DNA vaccination group and a normal vaccination group were significant enough and had a 95% confidence level.
Results/Discussion
Both trials concluded what was already proven and known, and unfortunately failed to show an improvement in the field. Both studies concluded that these vaccinations are safe. The trial focusing on the influenza virus proved that the vaccine is safe, and functions properly, in children. The study focusing on the influenza virus, unfortunately, failed to show that they should replace regular vaccinations in traditional clinics because they work just as effectively. The study with mice as subjects proved its safety, as well as proved that they are significantly different in effectiveness. However, the next step would be to conduct the shingles virus DNA vaccination study in humans. Since the first study conducted more directly on children and a recurring seasonal issue, this study should be taken more into consideration. Some vaccinations for viruses decrease in efficiency over time, although the exact time depends directly on the virus. DNA vaccinations cause the body to directly produce antigens for the pathogen.

As seen in section A (Houser et al. 2018) the data produced from the study doesn’t show a direct answer to the objective. The locations gave different results meaning there could be other unknown variables affecting results. The subjects in California, Wisconsin, and Texas saw a higher antibody response, however, those in Victoria didn’t. As I mentioned before, some vaccinations’ effectiveness decrease over time, and this study along, with the mice study, didn’t test the long term effect of their vaccine. Some parents are alarmed at the idea of vaccines because of the idea that a live virus is injected into their child. “DNA vaccines can be constructed to function with many encoded safety features while retaining the specificity of a subunit vaccine.” (Kutzler 2008) Parents often worry this will harm their child, but don’t need to worry as DNA vaccines are not proven to be safe These holes are the reason for a continuation of studies that. Holes that need to be addressed are the race and ethnicity of the child (environment), and the long term effectiveness of these DNA vaccines.
Conclusion
These studies help to inform researchers of the current information present, and areas where more manipulation needs to occur. These studies showed us a need to further research, because of a need for more information. Researchers take into account the current research when designing new experiments and will allow for better results in the future. All medical breakthroughs were tested and tested until they were ready to be spread leading to many saved lives. DNA vaccinations could be this next medical breakthrough, we just need to properly address, and test, it.
References
Houser, K. V., Yamshchikov, G. V., Bellamy, A. R., May, J., Enama, M. E., Sarwar, U., …Ledgerwood, J. E. (2018). DNA vaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: A phase 1 randomized clinical trial. PLoS ONE, 13(11), e0206837
Kim, A. R., Park, J., Kim, J. H., Kwak, J. E., Cho, Y., Lee, H., Jeong, M., Park, S. H., … Shin, E. \C. (2018). Herpes Zoster DNA Vaccines with IL-7 and IL-33 Molecular Adjuvants Elicit Protective T Cell Immunity. Immune network, 18(5), e38. doi:10.4110/in.2018.18.e38
Kutzler, M. A., & Weiner, D. B. (2008). DNA vaccines: ready for prime time?. Nature reviews. Genetics, 9(10), 776-88.
Lee, S. H., Danishmalik, S. N., & Sin, J. I. (2015). DNA vaccines, electroporation and their applications in cancer treatment. Human vaccines & immunotherapeutics, 11(8), 1889-900..


